Answer:
65.1 °C
Step-by-step explanation:
m = mass of the water = 0.5 kg
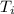 = initial temperature of water = 35.00 °C
= initial temperature of water = 35.00 °C
 = final temperature of water
= final temperature of water
c = specific heat of water = 4186 J/(kg °C)
Q = Amount of heat removed from water = 6.3 x 10⁴ J
Amount of heat removed from water is given as
Q = m c (
 -
-
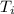 )
)
Inserting the values
6.3 x 10⁴ = (0.5) (4186) (
 - 35.00)
- 35.00)
 = 65.1 °C
= 65.1 °C