Answer : The enthalpy of the following reaction is, -390.3 KJ
Explanation :
The given balanced chemical reactions are,
(1)
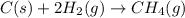
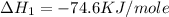
(2)
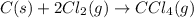
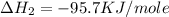
(3)
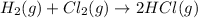
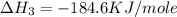
The final reaction of is,
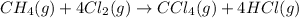
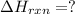
Now adding reaction 2 and twice of reaction 3 and reverse of reaction 1, we get the enthalpy of of the reaction.
The expression for enthalpy for the following reaction will be,
![\Delta H_(rxn)=[2* \Delta H_3]+[-1* \Delta H_1]+[1* \Delta H_2]](https://img.qammunity.org/2020/formulas/chemistry/high-school/d7bk0wqtmkg1etzxokqnfmcx2dxsuse826.png)
where,
n = number of moles
Now put all the given values in the above expression, we get:
![\Delta H_(rxn)=[2mole* (-184.6KJ/mole)]+[-1mole* (-74.6KJ/mole)]+[1* (-95.7KJ/mole)]=-390.3KJ](https://img.qammunity.org/2020/formulas/chemistry/high-school/81hgnrmdjio7pja0vyequnznnhfgcbb6da.png)
Therefore, the enthalpy of the following reaction is, -390.3 KJ