Answer:
oxygen atoms gain electrons and hydrogen atoms lose electron
Step-by-step explanation:
Hydrogen oxygen fuel cell involves redox reactions.
It is an electrochemical cell, which is used for many applications like rocket propellant.
The actual reaction is

Here hydrogen undergoes oxidation as it loses electrons
Oxygen undergoes reduction as it gains electrons.
The redox reactions are
At anode:
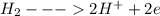 [loss of electrons by hydrogen]
[loss of electrons by hydrogen]
At cathode:
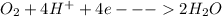 [gain of electrons by oxygen]
[gain of electrons by oxygen]