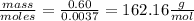Answer:
molar mass of nicotine will be 162.16g/mol
Step-by-step explanation:
The mass of nicotine taken = 0.60g
The volume of solution = 12mL
the osmotic pressure of solution = 7.55 atm
Temperature in kelvin =298.15K (25+ 273.15)
The formula which relates osmotic pressure and concentration (moles per L) is:
π = MRT
Where
π = osmotic pressure (unit atm) = 7.55 atm
M = molarity (mol /L)
T= temperature = (K) = 298.15 K
R = gas constant = 0.0821 L atm /mol K
Putting values
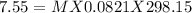
Therefore

Molarity is moles of solute dissolve per litre of solution
The volume of solution in litre = 0.012 L
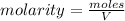
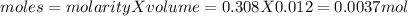
we know that
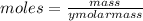
molar mass =