Answer: Option (d) is the correct answer.
Step-by-step explanation:
A chemical reaction in which heat energy is liberated is known as an exothermic reaction.
For example,
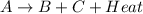
In an exothermic reaction, energy of reactants is more than the energy of products.
This means that potential energy of products is less than the potential energy of reactants.
Thus, we can conclude that in the reaction
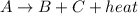 , the potential energy of the products is less than that of the reactant.
, the potential energy of the products is less than that of the reactant.