Answer:
The partial pressure of the
 in the final mixture is 200 kPa.
in the final mixture is 200 kPa.
Step-by-step explanation:
Pressure of nitrogen gas when the two tanks are disconnected = 500 kPa
Pressure of the carbon-dioxide gas when the two tanks are disconnected = 200 kPa
Moles of nitrogen gas =
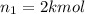
Moles of carbon dioxide gas =
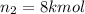
After connecting both the tanks:
The total pressure of the both gasses in the tank = p = 250 kPa
According to Dalton' law of partial pressure:
Total pressure is equal to sum of partial pressures of all the gases
Partial pressure of nitrogen =
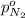
Partial pressure of carbon dioxide=
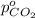
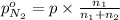
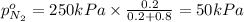
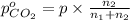
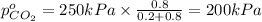
The partial pressure of the
 in the final mixture is 200 kPa.
in the final mixture is 200 kPa.