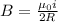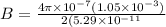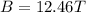Answer:
B = 12.46 T
Step-by-step explanation:
At n = 1 state we know that radius is given as
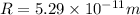
now we have
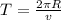
here we know that speed is given in that

now the time period is given as
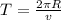
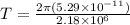
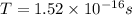
Now the electric current due to revolution of charge is given by
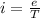
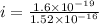
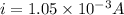
now magnetic field at the center position is given as