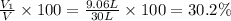Step-by-step explanation:
Mass of methane gas = 12 g
Mass of nitrogen gas = 1 g
Mass of carbon dioxide = 15 g
Volume of the container ,V= 30 L
Temperature of the gases,T= 25°C = 298.15 K=
a) Moles of methane gas:
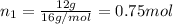
Moles of nitrogen gas:
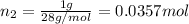
Moles of carbon dioxide gas:
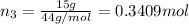
b) Partial pressure exerted by each gas.
The total pressure of the gases can be calculated by using an ideal gas equation:



P = 0.92 atm = Total pressure of the mixture
Partial pressure of all the gases can be determined by using Dalton's law of partial pressure
Partial pressure of methane gas
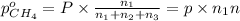
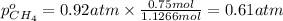
Partial pressure of nitrogen gas
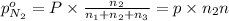
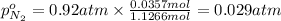
Partial pressure of carbon dioxide gas
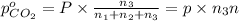
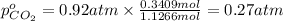
c) The total pressure exerted by the mixture is 0.92 atm.
d) Percentage by volume of each gas in the mixture
Volume of the methane gas:

Volume percentage of methane :
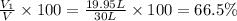
Volume of the nitrogen gas:
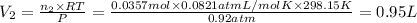
Volume percentage of nitrogen:
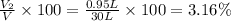
Volume of the carbon dioxide gas:

Volume percentage of carbon dioxide: