Answer:
The concentration of the potassium ions in the original potassium chromate solution is 4.2927 mol/L.
The concentration of the chromate ions in the final solution is 1.0731 mol/L.
Step-by-step explanation:
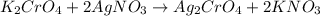
Volume of solution A i.e. solution of silver nitrate = 466.0 mL = 0.466 L
Volume of solution B i.e. solution of potassium chromate = 466.0 mL = 0.466 L
Moles of silver chromate =
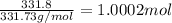
According to reaction , 1 mol of silver chromate is produce from 2 moles of silver nitrate.
Then, 1.0002 moles of silver chromate will be formed from:
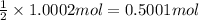 of silver nitrate.
of silver nitrate.
According to reaction , 1 mol of silver chromate is produce from 1 mole of potassium chromate.
Then, 1.0002 moles of silver chromate will be formed from:
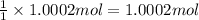 of potassium chromate
of potassium chromate
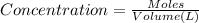
a) The concentration of the potassium ions in the original potassium chromate solution.
Volume of the original solution = 0.466 L
1 mol of potassium chromate dissociates into 2mol of potassium ions and 1 mol of chromate ions:
Moles of potassium ions = 2 × 1.0002 mol = 2.0004 mol
![[K^+]=(2.0004 mol)/(0.466 L)=4.2927 mol/L](https://img.qammunity.org/2020/formulas/chemistry/high-school/h0bq43vih2khsc5556b3ta4dnio3ne20h5.png)
b) The concentration of the chromate ions in the final solution
Volume of the final solution = 0.466 L + 0.466 L
Moles of chromate ions = 1 × 1.0002 mol = 1.0002 mol
![[CrO_4^(2+)]=(1.0002 mol)/(0.466 L+0.466L)=1.0731 mol/L](https://img.qammunity.org/2020/formulas/chemistry/high-school/o2zmmy3s7j426kclmvidjq5ol02xxg4teq.png)