Answer : The percent by mass of NaCl and KCl are, 18.11 % and 81.88 % respectively.
Explanation :
As we know that when a mixture of NaCl and KCl react with excess
 then the silver ion react with the chloride ion in both NaCl and KCl to form silver chloride.
then the silver ion react with the chloride ion in both NaCl and KCl to form silver chloride.
Let the mass of NaCl be, 'x' grams and the mass of KCl will be, (0.9440 - x) grams.
The molar mass of NaCl and KCl are, 58.5 and 74.5 g/mole respectively.
First we have to calculate the moles of NaCl and KCl.
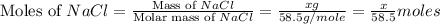
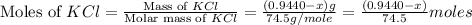
As, each mole of NaCl and KCl gives one mole of chloride ions.
So, moles of chloride ions in NaCl =
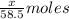
Moles of chloride ions in KCl =
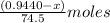
The total moles of chloride ions =
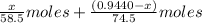
Now we have to calculate the moles of AgCl.
As we know that, this amount of chloride ion is same as the amount chloride ion present in the AgCl precipitate. That means,
Moles of AgCl = Moles of chloride ion =
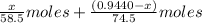
Now we have to calculate the moles of AgCl.
The molar mass of AgCl = 143.32 g/mole
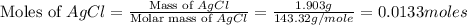
Now we have to determine the value of 'x'.
Moles of AgCl =
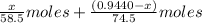
0.0133 mole =
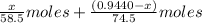
By solving the term, we get the value of 'x'.
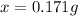
The mass of NaCl = x = 0.171 g
The mass of KCl = (0.9440 - x) = 0.9440 - 0.171 = 0.773 g
Now we have to calculate the mass percent of NaCl and KCl.
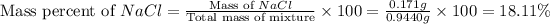
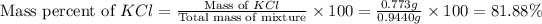
Therefore, the percent by mass of NaCl and KCl are, 18.11 % and 81.88 % respectively.