Answer: Option (b) is the correct answer.
Step-by-step explanation:
The given chemical reaction shows that hydrogen cyanide acid has been added to water which results in the formation of hydronium ion and cyanide ion.
Also, when we add a base like sodium hydroxide (NaOH) to HCN then it will help in accepting a proton (
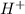 ) from hydrogen cyanide. As a result, formation of
) from hydrogen cyanide. As a result, formation of
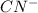 anion will be rapid and easy.
anion will be rapid and easy.
This will make the system not to do any extra work. So, amount of work done by system will decrease.
Thus, we can conclude that out of the given options, add solid NaOH to the reaction (assume no volume change) will decrease the amount of work the system could perform.