Answer:
The new pressure inside the container is 394.48 Pa.
Step-by-step explanation:
Given that,
Pressure = 550.0 kPa
Temperature = 25.0°C
Initial volume = 5.20 L
Final volume = 7.25 L
We need to calculate the new pressure inside the container
Using Boyle's law
PV = constant
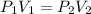
Where, P₁ = Pressure
V₁ = Initial volume
V₂ = Initial volume
Put the volume into the formula
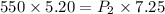
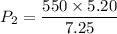
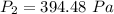
Hence, The new pressure inside the container is 394.48 Pa.