Answer: The mass of EDTA that would be needed is 24.3 grams.
Step-by-step explanation:
We are given:
Concentration of
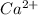 ions = 20 mg/L
ions = 20 mg/L
Converting this into grams/ Liter, we use the conversion factor:
1 g = 1000 mg
So,
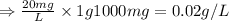
Now, we need to calculate the mass of calcium present in 44 gallons of drum.
Conversion factor used: 1 gallon = 3.785 L
So, 44 gallons = (44 × 3.785)L = 166.54 L
Calculating the mass of calcium ions in given amount of volume, we get:
In 1L of volume, the mass of calcium ions present are 0.02 g.
Thus, in 166.54 L of volume, the mass of calcium ions present will be =
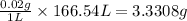
The chemical equation for the reaction of calcium ion with EDTA to form Ca[EDTA] complex follows:
![EDTA+Ca^(2+)\rightarrow Ca[EDTA]](https://img.qammunity.org/2020/formulas/chemistry/college/6e6635zv0efmqoacp8gsse6qce4qiuwm4b.png)
Molar mass of EDTA = 292.24 g/mol
Molar mass of
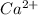 ion = 40 g/mol
ion = 40 g/mol
By Stoichiometry of the reaction:
40 grams of calcium ions reacts with 292.24 grams of EDTA.
So, 3.3308 grams of calcium ions will react with =
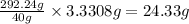 of EDTA.
of EDTA.
Hence, the mass of EDTA that would be needed is 24.3 grams.