Answer:
The effective molar mass of air at STP is 28.82 g/mol.
Step-by-step explanation:
At STP, the value of pressure is 1 atm.
At STP, the temperature is equal to 273.15 K
P = 1atm, T = 273.15 K
Density of the gas at STP ,d= 1.285 g/L
 (Ideal gas equation)
(Ideal gas equation)
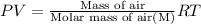

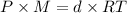
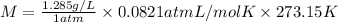
M = 28.81 g/mol
The effective molar mass of air at STP is 28.82 g/mol.