Answer:
The balanced chemical equation is

The conversions are
Molarity of HCl and volume gives us moles HCl
Moles HCl to moles
 (using mole ratio 1 : 1)
(using mole ratio 1 : 1)
Moles
 to Molarity
to Molarity

Molarity HCl = (moles solute HCl) / (volume of solution in L)
Rearranging the formula
We get moles HCl = Molarity × volume
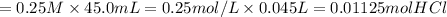
moles HCl = moles
 = 0.01125 mol
= 0.01125 mol

Molarity
 =(moles solute
=(moles solute
 ) / (volume of solution in L)
) / (volume of solution in L)
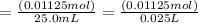
=0.450 mol/L or M (Answer)