Answer:
The final temperature of the combined metals is 49.2314 °C
Step-by-step explanation:
Heat gain by gold = Heat lost by iron
Thus,
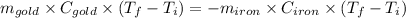
Where, negative sign signifies heat loss
Or,
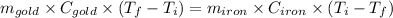
For gold:
Mass = 11.4 g
Initial temperature = 14.5 °C
Specific heat of gold = 0.129 J/g°C
For iron:
Mass = 18.4 kg
Initial temperature = 55.4 °C
Specific heat of water = 0.450 J/g°C
So,
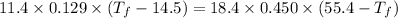
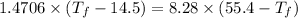
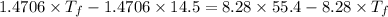
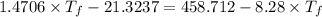
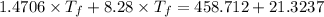
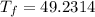
Thus,
The final temperature of the combined metals is 49.2314 °C