Answer:
Step-by-step explanation:
Given parameters:
Mass of ionic compound = 0.3257g
Mass of AgBr precipitate = 0.7165g
Unknown:
Percent mass of Br in the original compound.
Solution
The percent mass of Br in original compound =
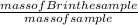
Now we have to find the mass of Br⁻:
We must note that the same mass of Br⁻ would move through the ionic sample to form the precipitate.
Mass of Br in AgBr =
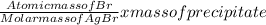
Mass of Br =
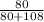 x 0.7165
x 0.7165
Mass of Br = 0.426 x 0.7165 = 0.305g
Percent mass of Br =
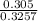 x 100 = 93.7%
x 100 = 93.7%