Answer :
In double-displacement (metathesis) reactions, such as precipitation and acid-base reactions, the cations of two reactants exchange places; these reactions are not redox processes.
In solution, single-displacement reactions occur when an atom of one element displaces the atom of another. Since one of the reactants is an element, all single-displacement reactions are redox processes.
Explanation :
Double-displacement reaction : It is defined as the reaction in which the cation of two reactants molecule exchange their places to give two different products.
The double-displacement reaction are not a redox reaction.
It is represented as :
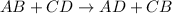
Where A and C are the cations and B and D are the anions.
Single-displacement reaction : It is a type of reaction in which the more reactive metal displaces the least reactive metal from its solution.
It is represented as :

Where A is the most reactive metal and BC is the compound in which the B is the least reactive metal which is displaced by the most reactive metal A.
The single-displacement reaction are a redox reaction.