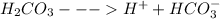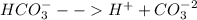Answer:
The incorrect about reasoning is that there is hydrogen ion present in the solution of dissolved carbon dioxide in water.
Step-by-step explanation:
The acid ph is less than 7.
If we have a solution of carbon dioxide it means we have dissolved carbon dioxide gas in water and this leads to reaction of carbon dioxide gas with water to produce carbonic acid.

This carbonic acid formed dissociates into carbonates and proton.
The formation of proton (hydronium ion) results the acidic pH of the solution.