Answer: 160 ml
Step-by-step explanation:
The expression used will be :
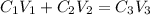
where,
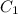 = concentration of Ist alcohol solution= 10%
= concentration of Ist alcohol solution= 10%
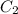 = concentration of 2nd alcohol solution= 55%
= concentration of 2nd alcohol solution= 55%
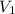 = volume of Ist alcohol solution = 200 ml
= volume of Ist alcohol solution = 200 ml
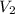 = volume of 2nd alcohol solution= v ml
= volume of 2nd alcohol solution= v ml
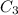 = concentration of resulting alcohol solution= 30%
= concentration of resulting alcohol solution= 30%
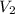 = volume of resulting alcohol solution= (v+200) ml
= volume of resulting alcohol solution= (v+200) ml
Now put all the given values in the above law, we get the volume of added.
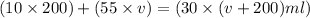
By solving the terms, we get :
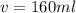
Therefore, the volume of 55% mixture needed to be added to obtain the desired solution is 160 ml.