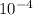Answer:
Step-by-step explanation:
1) Write the chemical equation for the solubility equilibrium:
- Ag₂ CrO₄ ⇄ 2 Ag⁺ + CrO₄²⁻
2) Write the expression for the solubility product constant, Ksp:
3) Relate the solubility with the concentrations:
4) Substitute in the Ksp equation:
- 1.10 × 10⁻¹² = s² × s = s³
5) Solve for s:
![s=\sqrt[3]{(1.10)(10^(-12))}=1.03](https://img.qammunity.org/2020/formulas/chemistry/middle-school/5tk0vi4mlmikq4dxnu6kxpenkp3gnfmdhy.png) ×
×