Answer:
(C) 0.220 M
Step-by-step explanation:
Given parameters:
volume of the dillutant = 250mL = 0.25L
Molarity of Na₂SO₄ before being diluted = 0.55M
Volume of Na₂SO₄ after dilution = 1.25L
Unknown:
Molarity of Na⁺ ions in the diluted solution
Solution
We can simply adopt the mole concept in solving this dilution problem. During dilution, we intend to reduce the concentration of a particular solution by increasing its volume. Therefore, we have to find the new concentration of the diluted Na₂SO₄. From here, we can be able to find the molarity of the sodium ions in the diluted solution:
Using dilution equation:
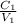 =
=
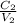
we can solve for the molarity of the diluted solution
C₁ = concentration of the original solution
V₁ = volume of the original solution
V₂ = final volume of the diluted solution
C₂ = final volume
We are solving of C₂:
Making C₂ the subject of the formula gives:
C₂ =
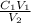
Therefore:
C₂ =
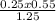 = 0.11M
= 0.11M
The diluted concentration is now 0.11M
To find the molarity of Na⁺ in Na₂SO₄:
We write the ionic form of the compound in solution:
Na₂SO₄ → 2Na⁺ + SO₄²⁻
In this solution,
1 mole of Na₂SO₄ will produce 2 moles of Na⁺ ions
From 0.11M of Na₂SO₄, we would have (2 x 0.11M) = 0.22M of Na⁺
The molarity of sodium ions will be 0.22M