Answer:
2412 psi is the pressure of the gas mixture in the tank.
Step-by-step explanation:
Pressure of the helium gas = 1,906 psi
Volume of the helium gas = 7.25 L
Pressure of the oxygen gas = 506 psi
Volume of the oxygen gas = 7.25 L
After mixing both gases in a container with volume 7.25 L at with constant temperature.
Since, the temperature and volume remained constant, pressure becomes directly dependent on moles of gases.So, when we mix gases together the moles of gases will also add and along with that pressure of individual gas will also get added to give total pressure of the mixture in a tank.
Total pressure = pressure(Heluim)+pressure(oxygen)
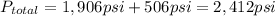
2412 psi is the pressure of the gas mixture in the tank.