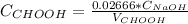Answer:
1. Multiply the equivalence point volume by the NaOH concentration.
2. Divide that result by the volume taken from the formic acid solution for titration.
Step-by-step explanation:
The reaction between NaOH and formic acid is one to one, it means that a NaOH mol reacts for each mole of formic acid in the solution. So the number of moles who react in the NaOH titrator is the same number of moles that were in the volume taken of the formic acid solution for titration. So, the number of formic acid moles can be calculated as:
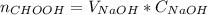
Then, divide that number of moles by the volume taken from the formic acid solution:
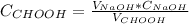
The concentrations must be in Molar units (Mol/Liter) and the volume in liters, so: