Answer:
The Balmer series refers to the spectral lines of hydrogen, associated to the emission of photons when an electron in the hydrogen atom jumps from a level
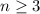 to the level
to the level
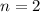 .
.
The wavelength associated to each spectral line of the Balmer series is given by:
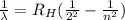
where
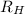 is the Rydberg constant for hydrogen, and where
is the Rydberg constant for hydrogen, and where
 is the initial level of the electron that jumps to the level n = 2.
is the initial level of the electron that jumps to the level n = 2.
The first few spectral lines associated to this series are withing the visible part of the electromagnetic spectrum, and their wavelengths are:
656 nm (red, corresponding to the transition
 )
)
486 nm (green,
 )
)
434 nm (blue,
 )
)
410 nm (violet,
 )
)
All the following lines lie in the ultraviolet part of the spectrum. The limit of the Balmer series, corresponding to the transition
 , is at 364.6 nm.
, is at 364.6 nm.