Answer:
Step-by-step explanation:
1) Radioactive decay equation (given):
2) Half-life of the isotope:
As stated the concentration after the half-life time is 50%. So, you can use A = A₀ / 2 and solve for t:
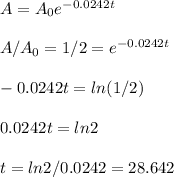
Thus, the answer, rounded to 3 decimal places as requested, is 28.642 years, and that is the half-life of the isotope.