Answer:
Step-by-step explanation:
1) Carbon-14 disintegration rate of a young (alive) tree: 0.266 per second per gram.
2) Carbon-14 disintegration rate of a wood sample prepeared from an object recovered at an archaelogical excavation (dead matter): 0.178 per second per gram.
3) Ratio of decay: A/A₀ = 0.178 / 0.266 = 0.669
4) Half-life equation: A/A₀ = (1/2)ⁿ, where n is the number of half-lives since the object died.
5) Substitute and solve for n:
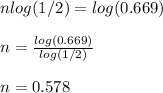
That means that 0.578 half-life has elapsed since the wood with which the object was created was dead matter.
6) Convert number of half-lives to years:
- 0.578 half-life × 5715 years/half-life = 3,303 years = 3.30 × 10³ years.