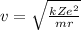Answer:
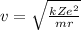
Step-by-step explanation:
The electrostatic attraction between the electron and the nucleus is given by Coulomb's Law
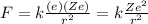
where we have
k is the Coulomb's constant
e is the charge of the electron
Ze is the charge of the nucleus
r is radius of the electron's orbit, which corresponds to the distance between the electron and the nucleus
The electron is moving by uniform circular motion, so the centripetal force acting on it is given by
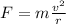
where
m is the mass of the electron
v is the speed of the electron
r is the radius of the orbit
Since the centripetal force is provided by the electrostatic attraction, we can write that the two forces are equal; so we obtain:
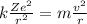
And re-arranging the equation for v we find: