Answer:
1.26 x 10²³atoms
Step-by-step explanation:
Given parameters:
Number of moles = 1mol
Unknown:
Number of atoms of sulfur = ?
Solution
From the given parameters, we can find the number of moles of sulfur in the given compound FeSO₄.
We use the formula mass of atoms to find the number of moles:
Atomic number of Fe = 56g
S = 32g
O = 16g
Formula mass = 56 + 32 + 4(16) = 152
Number of moles of sulfur =
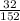 x 1
x 1
= 0.21mol
Using the number of moles of sulfur, we can now calculate the number of atoms:
Number of atoms = number of moles x 6.02 x 10²³
Number of atoms = 0.21 x 6.02 x 10²³ = 1.26 x 10²³atoms