Answer: contain different amounts of energy
Step-by-step explanation:
The energy
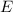 of a photon is given by:
of a photon is given by:
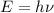
Where:
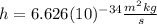 is the Planck constant
is the Planck constant
 is the frequency of the light which is inversely related to the wavelength.
is the frequency of the light which is inversely related to the wavelength.
Now, if we have photons of different light waves, this means we have photons with different frequencies.
As the energy of the photon depends on its frequency:
Photons of different light waves contain different amounts of energy.