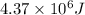Answer : The amount of heat required is, (C)
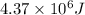
Explanation :
Formula used :
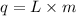
where,
q = heat required = ?
L = specific latent heat of fusion of ice =
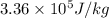
m = mass of ice = 13.00 kg
Now put all the given values in the above formula, we get:
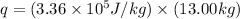
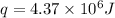
Therefore, the amount of heat required is,