Answer:
The enthalpy of the reaction of the chlorine with ozone is -162.5 kJ/mol.
Step-by-step explanation:
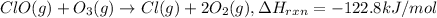 ...(1)
...(1)
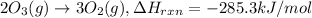 ...(2)
...(2)
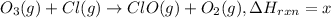 ...(3)
...(3)
The enthalpy of reaction for the reaction of chlorine with ozone can be calculated by using Hess's law:
(2 )- (1) = (3)
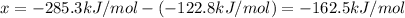
The enthalpy of the reaction of the chlorine with ozone is -162.5 kJ/mol.