Answer:
a)initial quality of water 0.724.
b)mass of water =2.45 kg
c)T=70°F
Step-by-step explanation:
h=732.5 Btu/lbm
h=763.33 KJ/kg (1 Btu=1.05 kj)
T=70°F⇒T=21.1°C
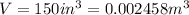
(a)From steam table
Properties of saturated steam at 21.1°C
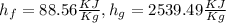
To find dryness fraction
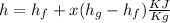
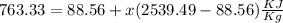
x=0.27
So initial quality of water 0.724.
(b)
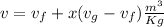
where v is specific volume
From steam table at 21.1°C
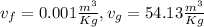
V=
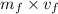
0.002458=
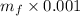
So mass of water =2.45 kg
(c)
Actually water will take latent heat ,it means that heating of will take place at constant temperature and constant pressure.So we can say that final temperature of water will remain same (T=70°F).