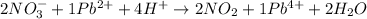Answer : The balanced reaction in acidic solution is,
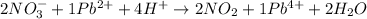
Explanation :
The given partial equation is,
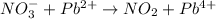
First we have to separate into half reaction. The two half reactions are:
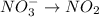
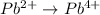
Now we have to balance the half reactions in acidic medium, we get:
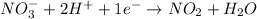 ............(1)
............(1)
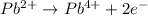 ............(2)
............(2)
Now we have to balance the electrons of the half reactions. When we are multiplying the equation (1) by 2, we get
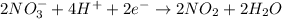 ...........(3)
...........(3)
Now we have to add both the half reactions (2) and (3), we get the final balanced chemical reaction.