Answer:
The rate of enzyme catalyzed reaction will increases by
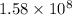 times.
times.
Step-by-step explanation:
According to the Arrhenius equation,
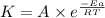
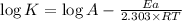
The expression used with catalyst and without catalyst is,
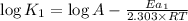 ...(1)
...(1)
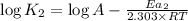 ...(2)
...(2)
On subtracting (2) from (1)
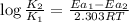
where,
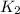 = rate of reaction with catalyst
= rate of reaction with catalyst
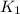 = rate of reaction without catalyst
= rate of reaction without catalyst
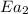 = activation energy with catalyst = 29.3 kJ/mol = 29300 J/mol
= activation energy with catalyst = 29.3 kJ/mol = 29300 J/mol
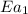 = activation energy without catalyst = 75.3 kJ/mol=75300 J/mol
= activation energy without catalyst = 75.3 kJ/mol=75300 J/mol
R = gas constant =8.314 J /mol K
T = temperature =
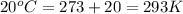
Now on substituting all the values in the above formula, we get
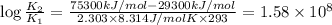
The rate of enzyme catalyzed reaction will increases by
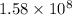 times.
times.