Answer:0.4061 L
Step-by-step explanation:
let initial volume of gas be
 =1 L
=1 L
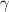 =1.3
=1.3
initial temprature
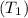 =273k
=273k
Now if gas is compressed adiabatically to half of its initial volume then
 =0.5L
=0.5L
Using adiabatic relation
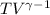 =constant
=constant
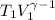 =
=
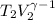
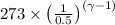 =
=

 =273
=273
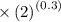
 =336.1k
=336.1k
Now the is cooled at constant pressure i.e.
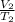 =
=
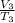
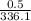 =
=
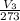
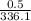 =
=
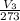
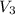 =0.406 L
=0.406 L