Answer:
The work required to compress the saturated water vapor to 895 kPa pressure is 130.9540 k J/Kg
Step-by-step explanation:
Given data in question
temperature = 140°C
pressure (P2) = 895 kPa
To find out
work required for compress saturated water
Solution
We know the equation for reversible work for compress saturated water vapor
i.e.
W =
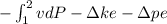
w is reversible work, v is specific volume, P is water vapor pressure and
ke is kinetic energy and pe is potential energy
and in question we have given v is constant so ke and pe will be zero
so
W =
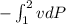
W = -v( P2 - P1 )
we can given in question temperature = 140°C and use steam table "A-4 saturated water - temperature table"
at this water property P1 will be 361.53 kPa and v will be 0.50850 m³/kg
so put these value in above equation
W = -0.50850( 104 - 361.53 )
W = 130.9540 kJ/Kg