Answer:
Part a)
Q = 3198 J
Part b)
It is compression of gas so this is energy transferred to the gas
Step-by-step explanation:
Part a)
Energy transfer during compression of gas is same as the work done on the gas
In isothermal process work done is given by the equation
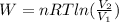
now we know that
n = 1.4 moles
T = 27 degree C = 300 K
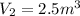
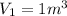
now we have
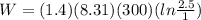
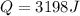
Part b)
It is compression of gas so this is energy transferred to the gas