Answer:
-25.63°C.
Step-by-step explanation:
We know that throttling is a constant enthalpy process
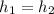
From steal table
We know that if we know only one property in side the dome then we will find the other property by using steam property table.
Temperature at saturation pressure 1 bar is 99.63°C and Temperature at saturation pressure 0.35 bar is about 74°C .
So from above we can say that change in temperature is -25.63°C.
But there is no any option for that .