Answer:
the atomic radius of a lithium atom is 0.152 nm
Step-by-step explanation:
Given data in question
structure = BCC
lattice constant (a) = 0.35092 nm
to find out
atomic radius of a lithium atom
solution
we know structure is BCC
for BCC radius formula is
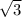 /4 × a
/4 × a
here we have known a value so we put a in radius formula
radius =
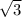 /4 × a
/4 × a
radius =
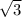 /4 × 0.35092
/4 × 0.35092
radius = 0.152 nm
so the atomic radius of a lithium atom is 0.152 nm