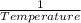Answer:
As the temperature goes down the solubility goes up.
Step-by-step explanation:
The solubility of the gas in the liquid is inversely proportional to the temperature. This is because as the temperature increases, the kinetic energy of the gas also increases. Thus, the motion of the gas molecules increases and the gas molecules try to escape the liquid.
Mathematically we can write this as,
Solubility ∝