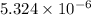Answer: The
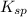 for calcium hydroxide is
for calcium hydroxide is
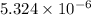
Step-by-step explanation:
To calculate the concentration of acid, we use the equation given by neutralization reaction:
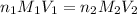
where,
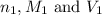 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is
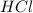
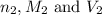 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is
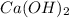
We are given:
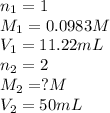
Putting values in above equation, we get:
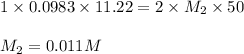
The concentration of
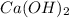 comes out to be 0.011 M.
comes out to be 0.011 M.
The balanced equilibrium reaction for the ionization of calcium hydroxide follows:
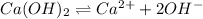
The expression for solubility constant for this reaction follows:
![K_(sp)=[Ca^(2+)][OH^-]^2](https://img.qammunity.org/2020/formulas/chemistry/college/7nky2s7c80f0u39z9mkn8ij7t0zchfjo9y.png)
Putting the values in above equation, we get:
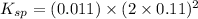
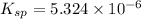
Hence, the
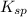 for calcium hydroxide is
for calcium hydroxide is