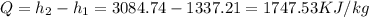Answer:1747.53KJ/kg
Step-by-step explanation:
Given data
Initial Temprature
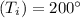
Quality
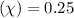
Final pressure
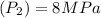
Given tank is rigid therefore specific Volume remains same
now calculating properties at
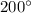
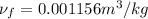
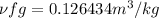
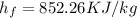
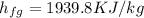
Now Specific volume of steam at
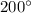
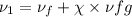
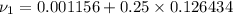
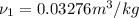
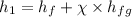
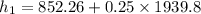
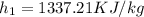
Now

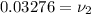
At 8 MPa and
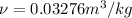
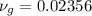
Since
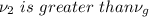
therefore final state of steam is superheated at
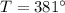
at this state
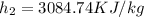 (obtained from steam table)
(obtained from steam table)
Therefore heat added