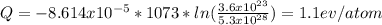Answer:
The energy for vacancy formation in silver is 1.1 ev/atom
Step-by-step explanation:
The total number of sites is equal to:
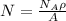
Where
NA = Avogadro´s number = 6.023x10²³atom/mol
A = atomic weight of silver = 107.9 g/mol
ρ = density of silver = 9.5 g/cm³
Replacing:
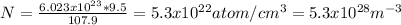
The energy for vacancy is equal:
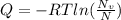
Where
R = 8.314 J/mol K = 8.614x10⁻⁵ev/atom K
T = 800°C = 1073 K
Nv = number of vacancy = 3.6x10²³m⁻³
Replacing: