Answer:
So heat transfer is 696 kJ
Step-by-step explanation:
Given:
K = 1.4
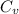 = 0.716 kJ/kg
= 0.716 kJ/kg
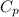 = 1 kJ/kg
= 1 kJ/kg
R = 0.287 kJ/kg
V = 1

P = 1000 kPa
T = 1000 K
Work delivered, δW = 696 kJ
It is isothermal process, so the initial and final temperature are same, that is T₁ = T₂ and the internal energy is zero (dU =0)
Therefore from 1st law of thermodynamcis,
δQ = dU + δW
= 0 + 696
= 696 kJ
So heat transfer is 696 kJ