Answer:
(b)False
Step-by-step explanation:
We know that specific Internal energy of gas u=
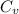 T
T
and specific enthalpy of gas h=
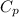 T
T
If we take the case of air we know that
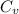 =0.707 KJ/Kg=K ,
=0.707 KJ/Kg=K ,
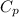 =1.005 KJ/Kg=K
=1.005 KJ/Kg=K
If we take A fixed temperature T=300 K
so u=212.1 KJ/ kg ,h=301.5 KJ/kg
So we can say that specific enthaply of gas is always greater than its specific internal energy.