Answer:
a) w= -95.53 KJ
b)Q= -95.53
Step-by-step explanation:
At initial condition
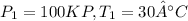
At final condition
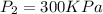
a)
It is constant temperature process so PV=C
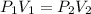
So work in constant temperature process
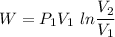 or
or
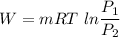
For air
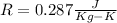
T is temperature in Kelvin.
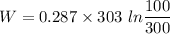 KJ
KJ
So w= -95.53 KJ ,This is work for unit mass.
Negative sign indicates , it is compression process.
b)
If take air as ideal gas then We know that internal energy for ideal gas is a function of temperature only.Here process is a constant temperature process so temperature of air will remains constant so ,change internal energy of air is equal to zero.
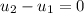
c)
From first law of thermodynamics
Q=
 +W
+W
Here
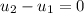
So Q=W ( w= -95.53 KJ )
⇒Q= -95.53 KJ
Negative sign indicates that heat flows out of the air.