Answer:
Total work done ,W = 437.5 kJ
Step-by-step explanation:
The area under the curve in the PV diagram gives the total work done.
As from the graph, we can find both the area of the trapezium and the area of the rectangle and then sum them to get the total area which is nothing but the total work done under the pressure volume graph when the air in the cylinder is slowly heated.
Therefore, we get
Area = 1 / 2 ( area of the trapezium ABCFA) + area of the rectangle FCDEF
=
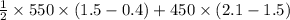
= 437.5 kJ