Answer:
The process is not possible.
Step-by-step explanation:
We know for ideal condition, the work done for isothermal process is
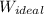 =
=
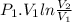
and for ideal gas, we know PV = mRT
Therefore,
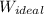 = mRT
= mRT
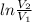
= mRT
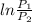
= 0.287 x 400
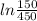
= -126.12 kJ/kg (negative sign indicates that the process is compressive, so work input to the compressor is 126.12 kJ/kg )
Now we know for adiabatic compression process
P
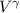 = C
= C
We know
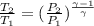
 = 556 K
= 556 K
For adiabatic work done,
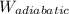 =
=
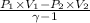
=
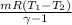
=
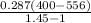
= -99.49 kJ/kg (negative sign indicates that the process is compressive, so work input to the compressor is 99.49 kJ/kg )
We know that in isothermal process, work input to the compressor is minimum. But in the above adiabatic polytropic process, work input to the compressor is less than the work done in the isothermal process.
Thus the process is not possible.