Answer:
(a) 74.023 psi (b) 133.4 KJ
Step-by-step explanation:
We have given m=1.2 lbm
p₁=90 psi
T₁=160°F=459.67+160=619.67
T₂=50°F=459.67+50=509.67
(a) We know that for constant volume pressure is directly proportional to temperature
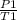 =
=
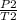
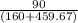 =
=
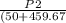
P₂=74.023 psi
(b) for R-134a
At P₁=90 psi ,T₁=160°F
h₁=458.658 KJ/KG (from the table)
At P₂=74.023 psi, T₂=50°F
h₂=219.593b (from the table)
m=1.2 lbm=.5443 kg
Q=.5443 ×(458.658-213.533)
Q=133.4 KJ